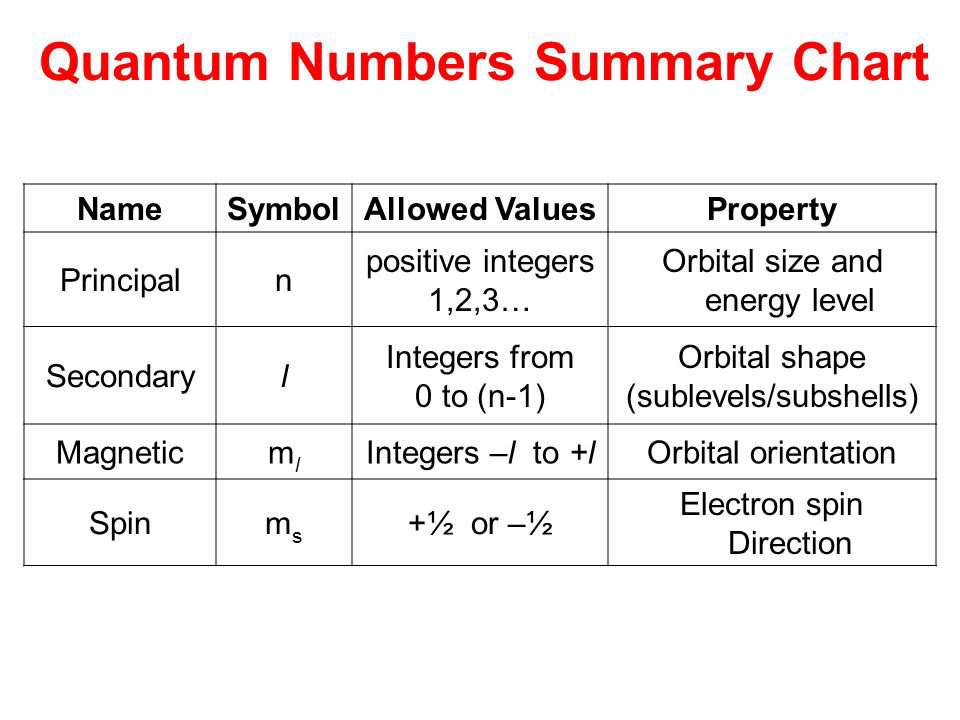
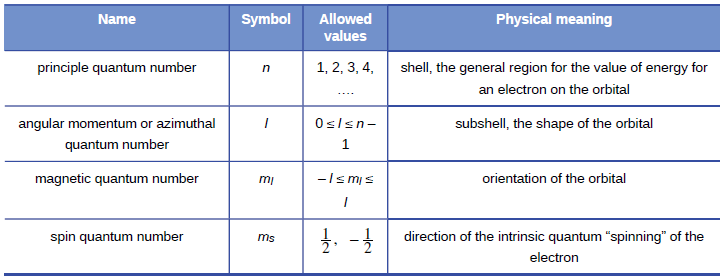
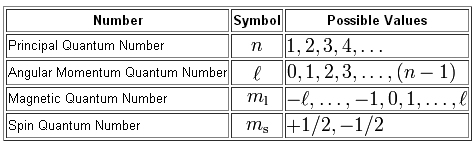
Property Principal Quantum Number
In quantum mechanics the principal quantum numbersymbolized n is one of four quantum numbersassigned to each electronin an atomto describe that electrons state. Principal quantum number It tells the principal energy level or shell to which the electron belongs.
Chemistry Life The Universe And Everything
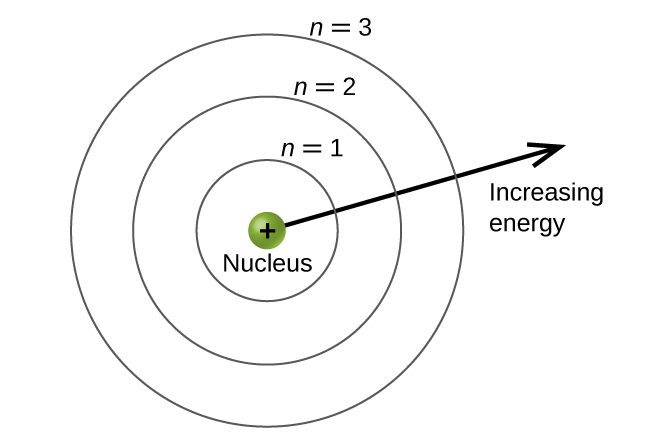
The lowest-energy state has n 1 the first excited state has n 2 and so on.
Property principal quantum number. The principle quantum number n describes the energy and distance from the nucleus and represents the shell. We define n to be the principal quantum number that labels the basic states of a system. The principal quantum number signified by n is the main energy level occupied by the electron.
It was given by Niels Bohr. The next quantum number the azimuthal quantum number denoted l describes the. For example the 3d subshell is in the n3 shell the 2s subshell is in the n 2 shell etc.
The principal quantum number n describes the energy of an electron and the most probable distance of the electron from the nucleus. The possible values for n are integers. This number cannot be zero and orbital sizes increase with increasing.
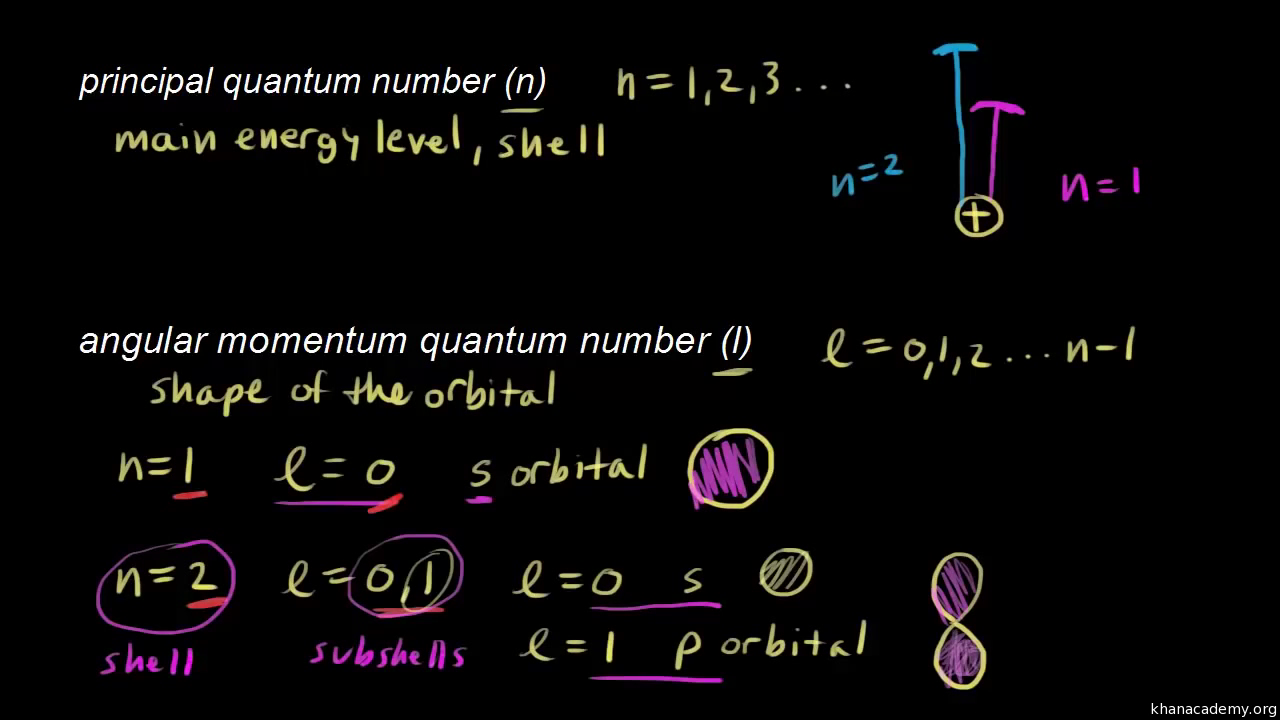
There are eight main shells referring to the principal quantum number n 12345678 that describes atomic orbitals. N 1 2 3 Specifies the energy of an electron and the size of the orbital the distance from the nucleus of the peak in a radial probability distribution plot. The number of subshells or l describes the shape of the orbital.
Its values are natural numbersfrom 1 making it a discrete variable. The principal quantum number n may fall within the range of positive integers excluding zero constrains the other quantum numbers. Energy levels are fixed distances from the nucleus of a given atom.
N denotes the energy level of each orbital. The principal quantum number n cannot be zero. The Principal Quantum Number The first quantum number describes the electron shell or energy level of an atom.
They are described in whole number increments eg 1 2 3 4 5 6. The spatial orientation of the orbital. The allowed values of n are therefore 1 2 3 4 and so on.
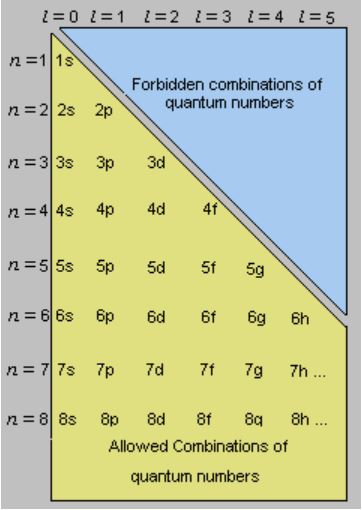
There are four major subshells. For example the azimuthal quantum number l can take whole number values from 0 to n 1. Thus the allowed values for the principal quantum number are n 1 2 3.
The average distance of the most electron-dense regions from the nucleus. The number of electrons. The first property describing the orbital is the principal quantum number n which is the same as in Bohrs model.
According to the current model of the atom it has a central nucleus with electrons orbiting around. The value of n ranges from 1 to the shell containing the outermost electron of that atom. S p d and f whose names derive from spectroscopic descriptions of sharp principal diffuse and fundamental.
Principal Quantum Number n. Click Here to Learn About the Different Types of Quantum Numbers. To understand it better.
There are three types of quantum numbers. The various principal energy shells are also designated by the letters KLMNOstarting from the nucleus. If n 3 for example l can be either 0 1 or 2.
In other words it refers to the size of the orbital and the energy level an electron is placed in. The magnetic quantum number m l can have whole number values from l to l. The shape of the orbital.
Quantum numbers describe the orbitals in which electrons are found. For the hydrogen atom the energy state E n is equal to m e 4 2ℏ 2 n 2 h c R n 2 where m is the mass of the electron. The principle quantum number is related to.
Principal quantum number can have any positive integral value from one to infinity. All orbitals that have the same value of n are said to be in the same shell level. The angular quantum number l can be any integer between 0 and n - 1.
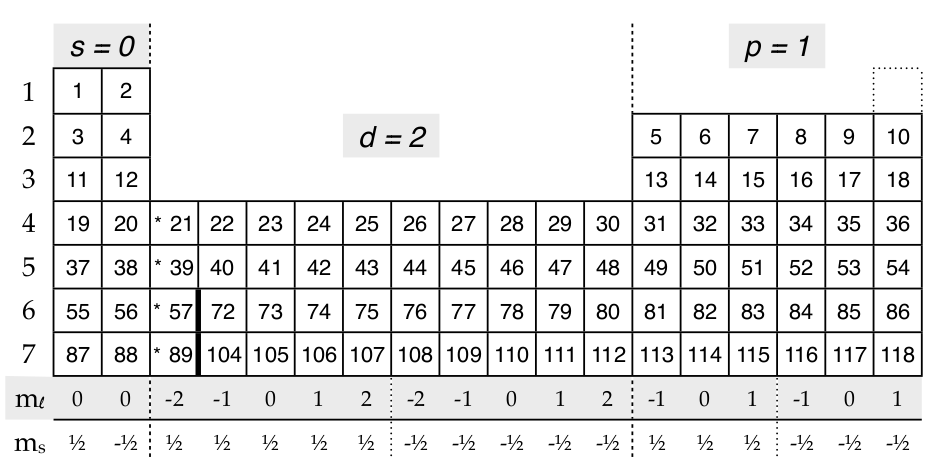
The main quantum number identified by n gives information about the orbital energy level of the electron. The four quantum numbers are the principle quantum number n the angular momentum quantum number l the magnetic quantum number m_l and the electron spin quantum number m_s. These orbits are located at different energy levels that are related to the distance from the electron to the nucleus.
As n n n increases more electrons are permitted in the shell the electron is farther from the nucleus and the electron is bound more loosely to the atom. It is donated by the letter n and can have any integral value except 0 ie. The principal quantum number is an integer n that corresponds to the gross energy states of the atom.
The principal quantum number describes the size of the orbital. Quantum Numbers can be used to Describe the Trajectory and the Movement of an Electron in an Atom. The principle quantum number n n n represents the energy level of an electron.
The principal quantum number the angular quantum number and the magnetic quantum number.
 Chemistry Understand Electronic Configuration Studying Amino Amino
Chemistry Understand Electronic Configuration Studying Amino Amino
 Openstax General Chemistry Ch6 Electronic Structure And Periodic Properties Of Elements Top Hat
Openstax General Chemistry Ch6 Electronic Structure And Periodic Properties Of Elements Top Hat
 Quantum Numbers Introduction To Chemistry
Quantum Numbers Introduction To Chemistry
 Quantum Numbers Video Quantum Physics Khan Academy
Quantum Numbers Video Quantum Physics Khan Academy
 Quantum Numbers Principal Azimuthal Magnetic Videos And Examples
Quantum Numbers Principal Azimuthal Magnetic Videos And Examples
 1 3 Quantum Numbers Chemistry Libretexts
1 3 Quantum Numbers Chemistry Libretexts
 Quantum Numbers Atomic Orbitals And Electron Configurations
Quantum Numbers Atomic Orbitals And Electron Configurations
 Principal Quantum Number An Overview Sciencedirect Topics
Principal Quantum Number An Overview Sciencedirect Topics
 Quantum Numbers Principal Azimuthal Magnetic Spin Definition Detailed Explanation With Videos
Quantum Numbers Principal Azimuthal Magnetic Spin Definition Detailed Explanation With Videos
 Summary Of Quantum Numbers Of Electrons In Atoms Ppt Video Online Download
Summary Of Quantum Numbers Of Electrons In Atoms Ppt Video Online Download
 Atomic Structure And Periodicity Ppt Download
Atomic Structure And Periodicity Ppt Download
 Which Scientist Developed The Quantum Mechanical Model Of The Atom Ppt Download
Which Scientist Developed The Quantum Mechanical Model Of The Atom Ppt Download
 Quantum Number Orbitals Of An Atom Principal Azimuthal Magnetic And Spin Quantum Number Of An Atom S Atomic Theory Study Chemistry Physics And Mathematics
Quantum Number Orbitals Of An Atom Principal Azimuthal Magnetic And Spin Quantum Number Of An Atom S Atomic Theory Study Chemistry Physics And Mathematics
 2 2 Atomic Orbitals And Quantum Numbers Chemistry Libretexts
2 2 Atomic Orbitals And Quantum Numbers Chemistry Libretexts
 Quantum Numbers Principal Quantum Number Teaching Chemistry Science Chemistry Biology College
Quantum Numbers Principal Quantum Number Teaching Chemistry Science Chemistry Biology College
 Spin The Quantum Property That Should Have Been Impossible
Spin The Quantum Property That Should Have Been Impossible
